Low muscle tone as an unspecific diagnosis and label
Parents are often told that their children have low muscle tone and this is given as the reason for why the child has movement difficulties. Teachers use the term freely as an explanation for movement and behavioral difficulties experienced by children in their classrooms.
This "diagnosis" of "low muscle tone" is usually based on the observation of a child's movement and posture. The child may have difficulties with performing age appropriate tasks and have difficulty with postural control, as well as poor endurance. (Martin et al 2007)
Recently, a study conducted by Martin et al, attempted to characterise hypotonia in children. The researchers surveyed pediatric PTs and OTs regarding the characteristics of hypotonia. According to this study, children with hypotonia displayed the following characteristics: decreased strength, hypermobile joints, increased flexibility, rounded shoulder posture, poor attention and motivation, leaning on supports, decreased activity tolerance, and delayed motor skill development. (Martin et al 2007)
The assumption is made that these difficulties arise, at least in part from this poorly defined concept of "low muscle tone", and the assumption is also made that this low tone originates within the central nervous system in some undefined way.
In the face of emerging knowledge about the structure of muscles, the impact of hyperrmobility on movement function and the role of anxiety and avoidance in setting central drive, as well as the importance of muscle strength and endurance on a child's everyday function, perhaps it is time to abandon the use of the term low muscle tone and replace it with more precise description of the child's movement difficulties (task performance) and an analysis of the factors contributing to these difficulties (impairments).
Defining muscle tone
Muscle tone has been defined as the active response of a muscle to lengthening, resulting from stretch of the muscle spindle (intrafual muscle fibre) which leads to an increase in the activity of the extrafusal muscle fibres in the same and surrounding muscles.
Muscle tone in this sense can also be described as the readiness of the muscles for action including the ability to adapt to changes in length and the need to maintain steady force output, in rapidly changing circumstances.
A muscle's responsiveness to stretch is mediated by the sensitivity of the stretch receptors within the intrafusal muscle fibres. This sensitivity is set by the activity in the gamma motor neurons within the intrafusal muscle fibres. Gamma motor activity in turn is set by readiness for action signals arising from the cortex, basal ganglia and cerebellum.
This has been referred to as "central set" and is mediated by the cortex and related to the task and the context. (Jacobs and Horak 2007).
Evidence from numerous studies suggests that:
- The cortex is also involved in changing postural responses with alterations in cognitive state, initial sensory-motor conditions, prior experience, and prior warning of a perturbation, all representing changes in ‘‘central set.’’
- The cerebellar-cortical loop is responsible for adapting postural responses based on prior experience.
- The basal ganglia-cortical loop is responsible for pre-selecting and optimizing postural responses based on current context.
Extracted from Jacobs and Horak (2007)
This reflex response to stretching (elongation) of a muscle is mediated by short and long latency reflexes, with reflex loops through the spinal cord, the brainstem and cortex. The changes in alpha motor neuron activity leading to force producing contraction of the extrafusal fibres within the same and surrounding muscles is determined by the demands of the task at hand.
In the absence of a neurological disorder, applying a slow stretch to a relaxed muscle does not elicit any active contraction (or an EMG response) in the extrafusal fibres. However, when a muscle is actively contracting, stretch applied to the muscle influences the recruitment of motor units in the stretched, as well as in surrounding muscles. (See below.)
Muscle tone as the resistance to passive movement
The term muscle tone has also been used to denote the inherent amount of resistance to lengthening of relaxed muscles and associated fascial structures when a joint is moved.
Muscle tone has traditionally been tested by moving limb segments through range and judging the amount of resistance to the lengthening. A muscle is said to have low tone when there is no resistance to passive lengthening, or when it feels soft on palpation.
Therefore this use of the term muscle tone in this instance refers to the inherent stiffness in the muscles and associated fascia. Importantly, it is not influenced by underlying activity in the stretch reflex.
Any resistance to passive lengthening of a relaxed muscle is provided in part by the stiffness in the connective tissue structures in the muscles. The majority of the resistance is provided by the elasticity of the giant muscle protein titin within the muscle cells. (See below)
This passive stiffness in the muscular and fascial structures provides a degree of stability to the trunk and limbs.
Stiffness in the connective tissue
In many instances where a child has a "diagnosis" of low muscle tone, the more accurate description is actually "low connective tissue tone".
The many connective tissue structures within the musculoskeletal system provide the body with its basic inherent stability . In individuals with generalised joint hypermobility the connective tissue is more compliant than usual. This increased compliance affects the:
- Joint capsule and ligaments
- Tendons
- Fascial structures – myofascia, aponeuroses, inter-muscular septa and deep fascia
The stiffness within connective tissue is provided by the cross-links between connective tissue fibrils. Connective tissue stiffness is affected by a number of factors:
- Age – increased cross-links
- Genetic – people with joint hypermobility syndrome have more compliant connective tissue
- Loading – connective tissue strength and stiffness adapts to loading
In joint hyerpmobility syndrome, the increased compliance in the connective tissue means that the trunk and limb segments are less stable than usual, and require more active muscle contraction to maintain stability and move joints through range.
Muscle stiffness - the role of titin
Titin is a very large elastic protein that forms part of the muscle cell. As the muscle is lengthened it stretches, and is responsible for the recoil of the muscle as it shortens passively. It has been estimated that up to 70 % of the passive tension in muscle comes from titin. (Fukuda et al 2008)
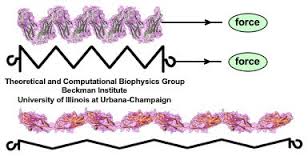
When the force is removed, the molecule recoils to its resting state. (Image ref www.ks.uiuc.edu/~ericlee/)
The titin isoform adapts to recent history by changing the degree of elasticity. It increases in size following repetitive loading, with an increase in the stiffness of the muscle.
Unloading (decreased activity) decreases the size of the titin isoform, with a decrease in passive resistance to lengthening (Udaka 2008). Muscle resistance to lengthening (ie the stiffness) is therefore affected by the history of loading and the strength of the muscle. Weak muscles have smaller titin isoforms and are therefore less stiff and provide less resistance to passive lengthening.
Here is the important message
If you want to increase tone, ie resistance to lengthening, you need to work on muscle strength.
Low muscle tone as a reduced readiness for action
Taking both the active and passive components of a muscle's (or group of muscles') readiness for action, it is time to redefine the term low muscle tone as a muscle's readiness for action which is influenced by the active and passive aspects of a muscle's response to lengthening or stretch.
The passive response is determined by the structure of titin within the muscle - this elasticity provides a degree of stability to the muscle in response to external perturbations, and is influenced by the configuration of the surrounding body segments and the forces acting on the body (Chiel et al 2009)..
The active response which depends on the sensitivity of the stretch reflex and can be viewed as readiness for action (Weedersteyn 2008).
Arousal and attention set the basic responsiveness of the system
The basic responsiveness of the neuromuscular system depends on many interacting factors including levels of arousal, the tendency to approach mediated by the dopamine system, motor planning and attention to the task at hand (Posner 2005).
Attention provides a funnel for sensory input that is important for an action or task. It determines whether sensory input is needed and important to the task at hand or the demands of the environment at any given time (Posner and Rothbart 2009).
Joint hypermobility syndrome and low connective tissue "tone"
Joint hypermobility syndrome is now a well recognised as an atypical configuration of the musculoskeletal system that may have an impact on a child's acquiring the basic movement skills.
Joint mobility is associated with a cautious temperament style as well as increased risk of chronic fatigue and chronic pain in children. It is also associated with dysautonomia and bladder problems in children and adults (Adib 2005). Children with JHS are also more prone to sports injuries (Pacey et al 2010).
The increased compliance in the connective tissues impacts on the joints, making them inherently less stable. More muscle action is therefore required to stabilise trunk and limb segments for postural and movement tasks.
Implications for practice
A careful assessments of a child's flexibility, muscle strength, endurance, and coordination will provide clues to reasons for the postural and movement difficulties that a child experiences. Once the measurable impairments are identified and the possible factors that have contributed to these impairments have been identified an effective training program that addresses impairments, function and participation can be implemented.
Recent updates on titin and muscle mechanics
Lieber, R. L., Roberts, T. J., Blemker, S. S., Lee, S. S. M., & Herzog, W. (2017). Skeletal muscle mechanics, energetics and plasticity. Journal of NeuroEngineering and Rehabilitation, 14, 108. http://doi.org/10.1186/s12984-017-0318-y
Herzog W. The multiple roles of titin in muscle contraction and force production. Biophys Rev. 2018 Jan 20. Abstract
Herzog, W. (2017). Skeletal muscle mechanics: questions, problems and possible solutions. Journal of NeuroEngineering and Rehabilitation, 14, 98. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5651624/
Tonino, P., Kiss, B., Strom, J., Methawasin, M., Smith, J. E., Kolb, J., … Granzier, H. (2017). The giant protein titin regulates the length of the striated muscle thick filament. Nature Communications, 8, 1041. http://doi.org/10.1038/s41467-017-01144-9
Bibliography and references
Adib, N., Davies, K., Grahame, R., Woo, P., & Murray, K. J. (2005). Joint hypermobility syndrome in childhood. A not so benign multisystem disorder? Rheumatology (Oxford, England), 44(6), 744-50
Chiel, H. J., Ting, L. H., Ekeberg, O., & Hartmann, M. J. Z. (2009). The brain in its body: motor control and sensing in a biomechanical context. The Journal of neuroscience : the official journal of the Society for Neuroscience, 29(41), 12807-14. Link to article
Duchateau, J., Semmler, J. G., & Enoka, R. M. (2006). Training adaptations in the behavior of human motor units. Journal of applied physiology (Bethesda, Md. : 1985), 101(6), 1766-75 Link to article
Gurfinkel, V., Cacciatore, T. W., Cordo, P., Horak, F., Nutt, J., & Skoss, R. (2006). Postural muscle tone in the body axis of healthy humans. Journal of neurophysiology, 96(5), 2678-87. Link to article
Harris, S. R. (2008). Congenital hypotonia: clinical and developmental assessment. Developmental medicine and child neurology, 50(12), 889-92. Link to article
Fukuda, N. et al., 2008. Physiological functions of the giant elastic protein titin in mammalian striated muscle. The journal of physiological sciences : JPS, 58(3), 151-9. PDF
Jacobs, J. V., & Horak, F. B. (2007). Cortical control of postural responses. Journal of neural transmission (Vienna, Austria : 1996), 114(10), 1339-48. Link to article
Mark Latash, Louise Wood and Dale Ulrich: What is currently known about hypotonia, motor skill development, and physical activity in Down syndrome
Martin, K., Inman, J., Kirschner, A., Deming, K., Gumbel, R., & Voelker, L. (2005). Characteristics of Hypotonia in Children: A Consensus Opinion of Pediatric Occupational and Physical Therapists. Pediatric Physical Therapy, 17(4), 275-282. Link to article
Pacey V, Nicholson LL, Adams RD, Munn J, Munns CF.(2010) Generalized joint hypermobility and risk of lower limb joint injury during sport: a systematic review with meta-analysis. Am J Sports Med. Jul;38(7):1487-97. Link to article
Pilon, Julie M.; Sadler, Gabrielli T.; Bartlett, Doreen J. (2000) Relationship of Hypotonia and Joint Laxity to Motor Development During Infancy. Pediatric Physical Therapy. 12(1):10-15 Abstract Research Gate request
Posner J, Russell JA, Peterson BS. (2005) The circumplex model of affect: an integrative approach to affective neuroscience, cognitive development, and
psychopathology. Dev Psychopathol. 17(3):715-34. PDF
Posner MI, Rothbart MK. (2009) Toward a physical basis of attention and self-regulation. Phys Life Rev. 2009 Jun;6(2):103-20 PDF
Sponberg, S., Libby, T., Mullens, C. H., & Full, R. J. (2011). Shifts in a single muscle?s control potential of body dynamics are determined by mechanical feedback. Philosophical Transactions of the Royal Society B: Biological Sciences, 366(1570), 1606-1620. Link to article
Strubhar, A. J., Meranda, K., & Morgan, A. (2007). Outcomes of infants with idiopathic hypotonia. Pediatric physical therapy : the official publication of the Section on Pediatrics of the American Physical Therapy Association, 19(3), 227-35. Request via Research Gate
Udaka, J., Ohmori, S., Terui, T., Ohtsuki, I., Ishiwata, S., Kurihara, S., et al. (2008). Disuse-induced preferential loss of the giant protein titin depresses muscle performance via abnormal sarcomeric organization. The Journal of general physiology, 131(1), 33-41. Link to article
Weerdesteyn, V., Laing, A. C., & Robinovitch, S. N. (2008). Automated postural responses are modified in a functional manner by instruction. Experimental brain research. 186(4), 571-80. Link to article
Wishart, J. (1999). Learning the hard way: Avoidance strategies in with Down ’ s syndrome. Cognitive Development, 1(2), 47-55. Link to article

